At the Centre for Industrial Rheology, we provide comprehensive interfacial characterisation services. For surface tension specifically, we utilise two key methods:
- Drop Shape Analysis, which primarily focuses on measurements after roughly 1 second of surface age.
- Bubble Pressure Tensiometry, which extends this analysis down to surface ages as short as 5 milliseconds onwards

Surface tension is a property that influences things such as how an ink forms a droplet to how a coating wets a surface. A common formulation challenge, however, is that this property is also highly sensitive to temperature. Ensuring surface tension behaves as expected across different temperatures is fundamental and can be the difference between a product that works and one that fails. We can characterise surface tension over a wide temperature range of -10 to 130 °C.
Contact us to discuss your requirements
Contact us
Introduction to Surface Tension
Surface tension is a property of liquids that arises directly from cohesive forces. Inside the bulk of a liquid, a molecule within the liquid is completely surrounded. It’s pulled equally in all directions by its neighbours, so all the forces balance out, and the net force is zero.
A molecule at the air-liquid interface is in a different situation, as it only has molecules beside and below it. Since there are no molecules above it to pull upward, it experiences a strong net inward pull from the molecules within the liquid. This collective inward pull forces the surface molecules tightly together, creating tension and minimising the liquid’s surface area.
The Influence of Temperature on Surface Tension
Surface tension and temperature share an inverse relationship. As the temperature increases, surface tension decreases. When a liquid is heated, its molecules gain kinetic energy and begin to move more vigorously. This increased kinetic energy directly counteracts the cohesive forces. With molecules in more rapid motion, intermolecular attractions are less effective, which, in turn, weakens the net inward pull experienced by the surface molecules. As a result, the tension at the liquid’s interface is reduced, and the surface tension drops.
How We Measure Surface Tension at Temperature 
We can measure surface tension across a wide temperature range using Bubble Pressure Tensiometry. This method is ideal due to its enclosed sample vessel, which helps to provide a stable thermal environment. In contrast, Drop Shape Analysis, another technique we utilise, features an open design. This lack of containment makes precise temperature control challenging.
Our bubble pressure tensiometer integrates seamlessly with our new high-precision thermal circulator. This powerful circulator provides exceptionally stable temperature management across a wide thermal fluid range of -10 to 130 °C.
This allows us to go far beyond simple ambient conditions to accurately simulate real-world conditions from sub-ambient environments to high-temperature processes.
Figure 2 below shows data collected for two different cooking oils, rapeseed oil and sunflower oil. Data can be captured at surface ages as short as 5ms, relevant for dynamic and fast processes where new interfaces are created rapidly. In cases where such analysis is not necessary, a simple equilibrated surface tension value can also be provided.
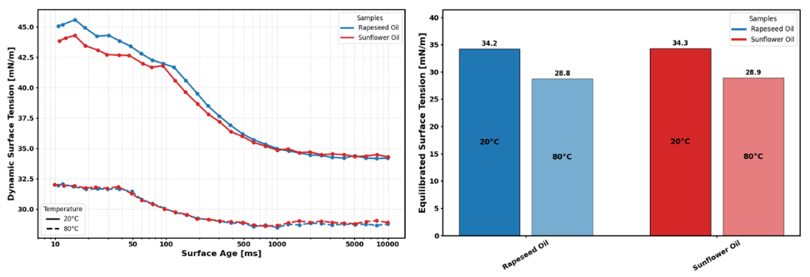
At 20 °C, both samples achieve a reduction in surface tension of roughly 10 mN/m by the time they reach a surface age of 1000ms. Increasing the temperature to 80 °C results in a significantly lower initial surface tension, which aligns with theoretical predictions. At this elevated temperature, the surface tension reduction is far less pronounced, with a reduction of around 3 mN/m over the same period. Comparison of equilibrated values offers a single, straightforward metric for comparing temperature effects on surface tension, independent of surfactant kinetics, with values of 34.2 mN/m at 20 °C for rapeseed oil and 28.8 mN/m at 80 °C.
There are many applications where surface tension characterisation over a range of temperatures is essential for truly understanding how a product may perform under real use. Some key examples include:
Performance Fluids, Heat Management Fluids, Coolants and Thermal Fluids
In systems where heat transfer and fluid circulation occur, such as automotive engines, electric vehicle battery packs, power electronics, or industrial heat exchangers, surface tension variation with temperature directly impacts system performance by influencing how the fluid wets components and dissipates hot spots.
Inkjet Printing, Spraying, and Atomisation
Inkjet processes are extremely sensitive to surface tension, with distinct targets for optimisation below 20 milliseconds of surface age. An optimal profile requires a careful balance, with initial surface tension being high enough to ensure meniscus stability at the nozzle, while a rapid subsequent reduction is needed to facilitate droplet formation and wetting. Temperature affects this profile, and as such, needs to be sufficiently characterised. For other general spraying and atomisation processes, the same rule applies as above.
Cleaning and Detergency
Heating a detergent lowers its surface tension, enhancing wetting and penetration into fabrics. It is therefore essential to ensure that surface tension at typical wash temperatures is desirably low to achieve the required wetting. The synergy between heat and surfactant kinetics plays a key role in achieving optimal wetting in detergency.
Summary
Understanding how a liquid’s surface tension behaves at its intended operating temperature is fundamental to ensuring effective product performance, from an engine coolant’s ability to wet surfaces to an ink’s ability to form a perfect droplet. The data presented here shows that temperature can significantly affect surface tension and surfactant adsorption kinetics.
At the Centre for Industrial Rheology, we have advanced instrumentation and in-depth expertise to accurately characterise your formulations across a wide range of temperatures. Contact us today to discuss your application and let us help you fully understand how your product’s surface tension changes with temperature

Related Articles;
Using Dynamic Surface Tension to Understand Household Surface Spray Performance
Nozzle to Substrate – Unlocking Ink Performance with Dynamic Surface Tension Measurements
The Role of Dynamic Surface Tension in Optimising Nasal Spray Performance
Wasif Altaf serves as an Applications Specialist at the Centre for Industrial Rheology, leveraging a chemical engineering background (BEng) to bridge theory and practice. His work focuses on advanced rheological characterisation.
