 Droplet Shape Analysis
Droplet Shape Analysis
Analysis of a droplet on a surface, also known as sessile drop analysis, allows for measurement of the angle at which a droplet of liquid interacts with a surface. The contact angle measured allows some conclusions to be drawn around the energetic interactions between the liquid, the surrounding air and the surface, the wettability of the solid i.e. how well a liquid interacts with the surface and, if water is the liquid used, the hydrophobicity or hydrophilicity (or polarity) of the surface.
If you would like to arrange a discussion about how our characterisation capabilities can help you, please feel welcome to contact us.
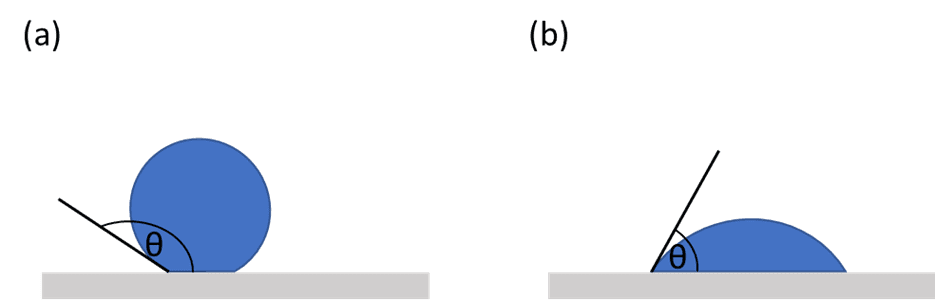
Contact angles can range from 0 to 180 degrees. When using water, if the contact angle is less than 90 degrees the material can be described as hydrophilic and if it is between 90 degrees and 180 degrees the material can be described as hydrophobic. Usually, the lower the contact angle the better the wettability of the material under study. Contact angles are all determined by the energetic interaction between the liquid and the surface, therefore it is possible to use the contact angle of a range of known liquids to determine the surface energy or characteristics of an unknown, or newly developed, material.
Contact angles are extensively used within the materials and textiles industry to showcase how treatment of a fabric or material can change the wetting properties, within the printing industry to show how likely an ink is to spread over a surface, within the nail polish industry to look at wetting of the nail polish onto the nail or, finally, the household cleaning industry to understand wetting or adhesion of a product to a surface. To showcase this interesting area of surface science we decided to analyse the interaction between water droplets and the surface of a leaf.
Droplet Shape on Leaves – Foliage is Power
Analysis of the interaction between liquid droplets and leaves can be useful for agrochemical formulation development as knowing the interaction between a formulation and a leaf surface can provide valuable information about adhesion, spray retention, risk of run-off and wettability.
 In this mini-study, we took a range of leaves from around the Centre of Industrial Rheology and subjected them to sessile drop analysis using our Krüss DSA-30R drop shape analyser. Leaves taken for analysis were: garden privet, grass, iris, ivy (Hedera helix spp. helix and Hedera helix spp. hibernica) and white dead nettle. In all cases the leaves were mounted onto the sample plate using double-sided tape, ensuring that the leaf was flat and natural roll-off by water droplets was avoided. Droplets were 5 µL in volume and the contact angle was measured for 60 seconds to allow for equilibration on the surface. The results were compared with PTFE, which is commonly used as a comparator for wetting experiments.
In this mini-study, we took a range of leaves from around the Centre of Industrial Rheology and subjected them to sessile drop analysis using our Krüss DSA-30R drop shape analyser. Leaves taken for analysis were: garden privet, grass, iris, ivy (Hedera helix spp. helix and Hedera helix spp. hibernica) and white dead nettle. In all cases the leaves were mounted onto the sample plate using double-sided tape, ensuring that the leaf was flat and natural roll-off by water droplets was avoided. Droplets were 5 µL in volume and the contact angle was measured for 60 seconds to allow for equilibration on the surface. The results were compared with PTFE, which is commonly used as a comparator for wetting experiments.
Results
Leaf-surface topography:
The garden privet leaves were elliptic and lightly waxed and had no hairs. Both the iris and grass were linear in shape. The iris leaf contained veins that ran along the length of the leaf whereas the grass leaf had one larger central vein. The iris leaf was waxy with no hairs, but the grass leaf had very small hairs covering the upper surface. Both ivy plants had leaves that were shiny, with a waxy surface. The leaves were either oblong in the case of spp. hibernica or hierarchically lobed in the case of spp. helix. Neither had any hairs. Finally, the white dead nettle leaf was cordate, with a serrated edge. The surface was soft and covered in very fine hairs.
Sessile drop analysis:
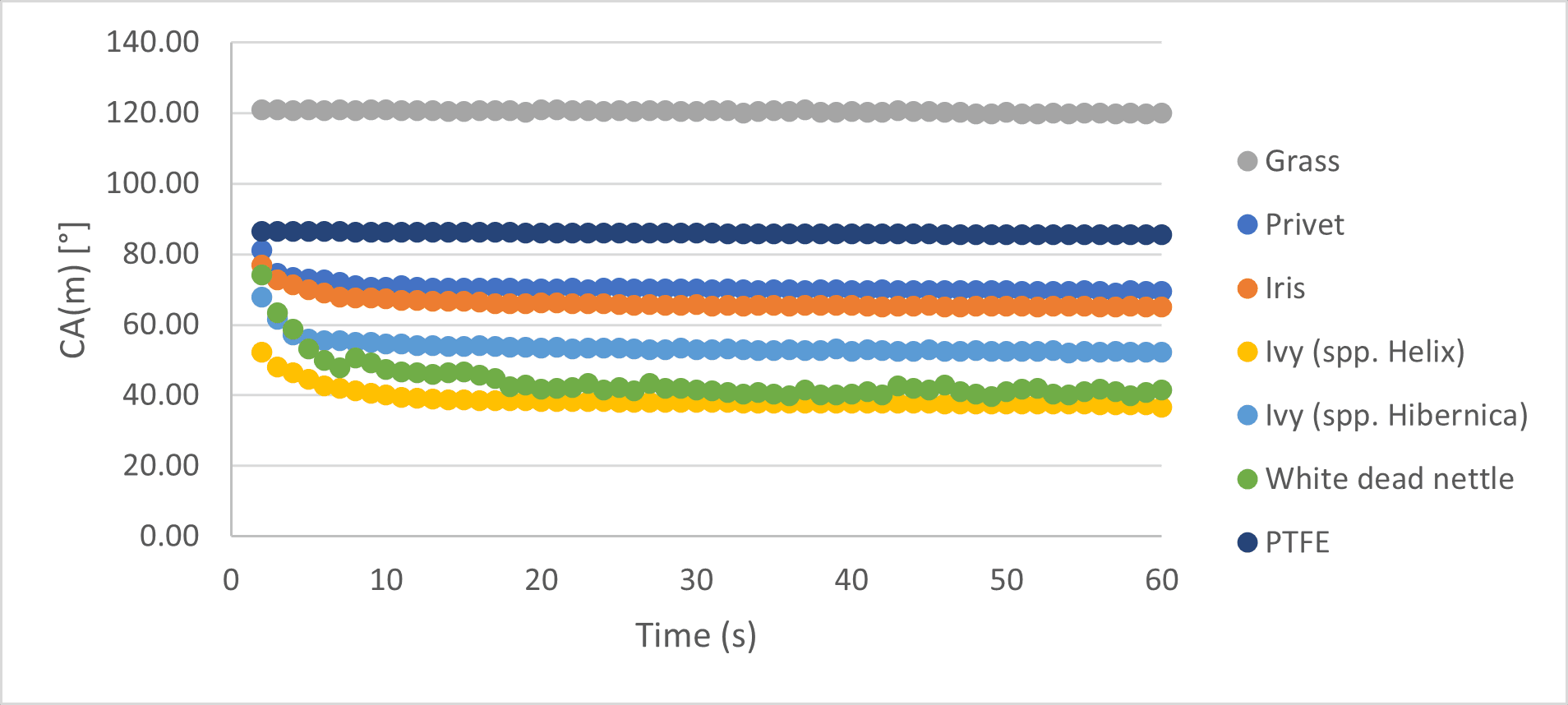
The majority of the leaves studied could be described as hydrophilic, with contact angles considerably smaller than 90 degrees. As a comparator, the contact angle for PTFE was 85.88°, which is used as the industry benchmark for hydrophobicity. Grass was an outlier with the highest contact angle of 120.41° and can therefore be described as hydrophobic. The plant with the most hydrophilic leaves was the ivy, spp. helix. The iris and privet had similar contact angles, although the leaf shape and topography were fairly different. Interestingly, the difference in contact angle between the two different ivy plants was large and suggests a significant difference in the hydrophobicity of the leaf surface. All of the leaves identified as hydrophilic exhibited some wetting behaviour, where the droplet ‘settled’ onto the leaf surface before reaching equilibrium. This is shown in the early stages of the experiment, and in the case of privet, both ivy species and the iris this completed within 6-7 seconds of the experiment starting. The clear exception to this was the white dead nettle leaf, where the wetting behaviour and equilibration took up to 15 seconds. This exception will be discussed further.
Finally, neither PTFE nor grass showed any ‘settling’ behaviour. The white dead nettle leaf itself showed some very interesting results. Under a microscope, the surface could be clearly seen to be covered in small hairs, which affected the droplet’s behaviour on the surface. Over the course of a few seconds the droplet settled onto the surface of the leaf and the hairs punctured the droplet, leading to an observed increase in wetting times. This behaviour was reminiscent of hemiwicking, which is defined as ‘the spreading of a liquid on a rough hydrophilic surface driven by capillarity’. It is important to state that hemiwicking is a surface process and is different to ‘wicking’, which would allow liquid to transfer through the bulk material away from the surface.
The contact angle between the liquid and the leaf can be used to draw conclusions about the surface energy of the leaf. A high contact angle, i.e. poor interaction between the leaf and the liquid, shows that the leaf has a low surface energy or there is a low chemical affinity between the leaf and the liquid. A low contact angle, i.e. good interaction between the leaf and the liquid, shows that the leaf has a high surface energy or there is a high chemical affinity between the leaf and the liquid.
The example showcased here has clear application within the agrochemicals industry, especially in relation to droplet roll-off during application and exploiting differences in hydrophobicity or leaf surface energy between different species. This work can easily be expanded to include a range of temperatures so mimicking real-world conditions, droplet sizes and surfaces. It could also provide valuable additional information when exploring different formulations allowing for targeting of specific plant species, which could be especially powerful when undertaking targeted dosing.
If you would like to arrange a discussion about how our characterisation capabilities can help you, please feel welcome to contact us.
