
Oral solid dosage (OSD) is a common method for delivering pharmaceutical drugs, nutraceuticals and supplements. In an OSD product, the active ingredient is dispersed among the excipient, or inactive components, which are powders. The excipient can have a range of roles, including stabilising and bulking-up the tablet, improving powder flowability during manufacture, preventing aggregation over the shelf-life or facilitating the mechanism of administration of the drug. For example, some excipients can aid drug absorption or enhance solubility. Understanding the powder-flow characteristics and behaviours of powders during manufacture can provide valuable insight into the most efficient ways of handling materials and provide a means for trouble-shooting any under-performing processes during production.
Contact us to arrange a lab tour and discuss rheological techniques with our experts

The most common forms for OSD products are tablets and capsules. In both cases, the OSD product will contain a mixture of the active ingredient(s) and excipient powder. The preparation of tablets and capsules are subtly different; tablets contain powders that are compressed into small pellets that are usually coated with another material, whereas capsules are created by filling a gelatine container with powder. Coating the tablets or encasing the powder by using capsules can be advantageous as it reduces dust, imparts a colour to the product and can impact upon processing in the body, for example by tuning the active ingredient release-rate.
In all cases, during mixing of excipient and active ingredient it is important to ensure that the components are evenly distributed so the dissolution profile and bioavailability are both good.
For information about dry powder inhalers, please see our article.
Powder flow: what is it and why should I care?
When working with powders there are numerous flow issues that can occur, which can impact upon the processing and manufacture of products. Common issues include funnelling, rat-holing, arching, caking and wall build-up, Figure 1. Some issues require additional cleaning of equipment between batches, but others may lead to a change in homogeneity of the mixture, therefore affecting the final formulation.
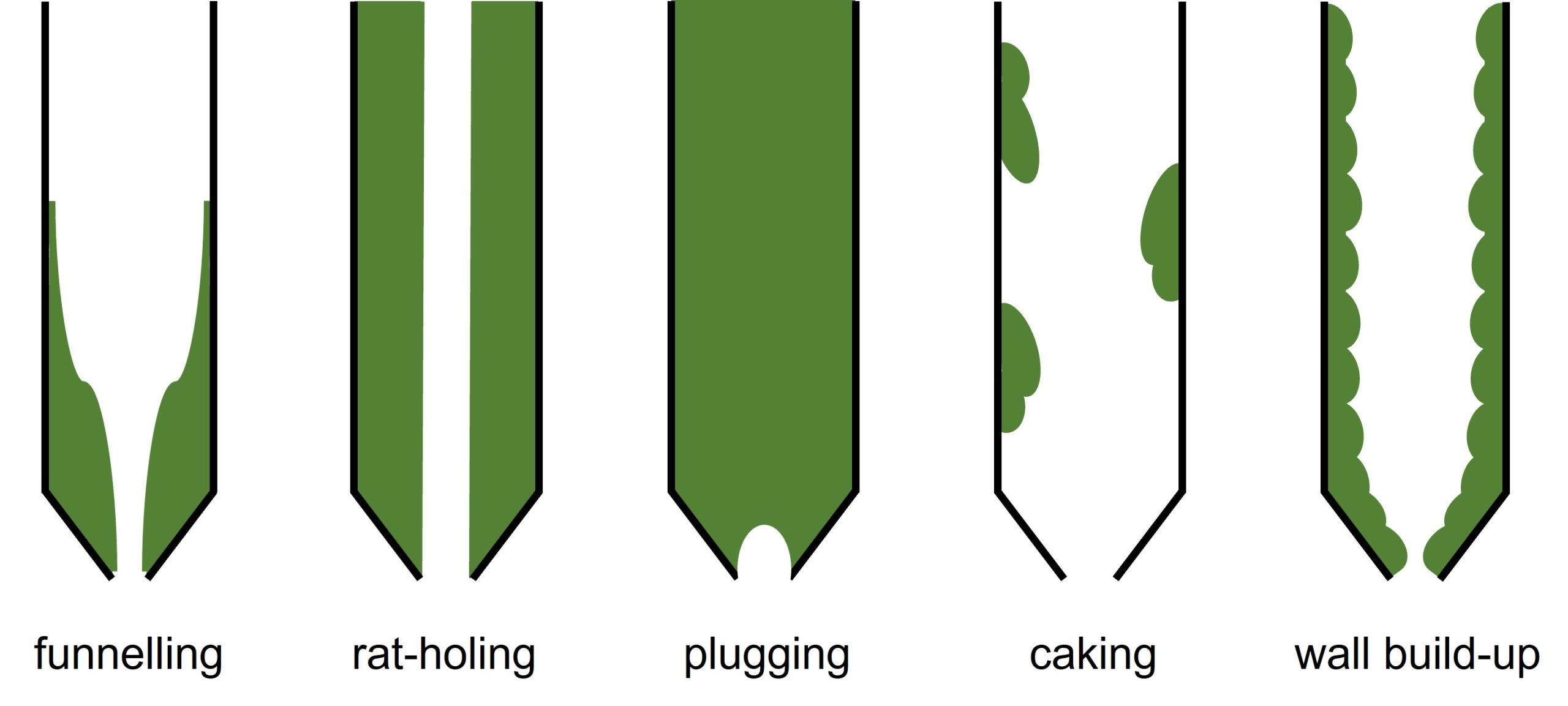
Powder-flow rheology can determine numerous parameters relating to powder behaviour. In the case of powders, it is important to collect multiple sets of data to build an overall picture of the powder’s behaviour and glean valuable insights. There are three principal areas for testing, each of which can be further sub-divided into component tests for interrogating that particular parameter:
-
Static tests
Used for measuring the properties of a powder when it is still and how much energy is required to induce movement in the powder body.
- Compressibility
- Shear cell measurements
- Wall-friction measurements
-
Dynamic tests
Used for measuring the properties of a powder once it is flowing, and how much energy is required to maintain movement of the powder body.
- Basic flowability energy
- Specific energy
- Consolidation
-
Aeration tests
Used to measure how easily a powder can be fluidized using air, and how the air interacts with the powder body.
- Permeability of the powder to air
- Aeration and how much air is required to induce fluidization
Powder flow and OSD
There are four commonly used processes for manufacturing OSD used within the pharmaceutical industry. These, and rheological techniques that can be used to monitor powder behaviour during these processes, are outlined below.
1. Wet Granulation
During this process, solids and liquids are either combined by high or low shear mixing, or the powder is aerated using a fluid-bed mixer and sprayed with the solvent, sometimes containing a binding agent, to encourage agglomeration of granules, Figure 2. Solvents commonly used are water, ethanol, isopropanol and dichloromethane. Once agglomeration is complete the granules are dried either in an oven, by using a fluid-bed drier, a microwave or a vacuum. Milling is the final step that is required and is to remove any lumps and generate a homogeneous powder. The output is a dense granule that can be compressed or encapsulated to give the final drug.
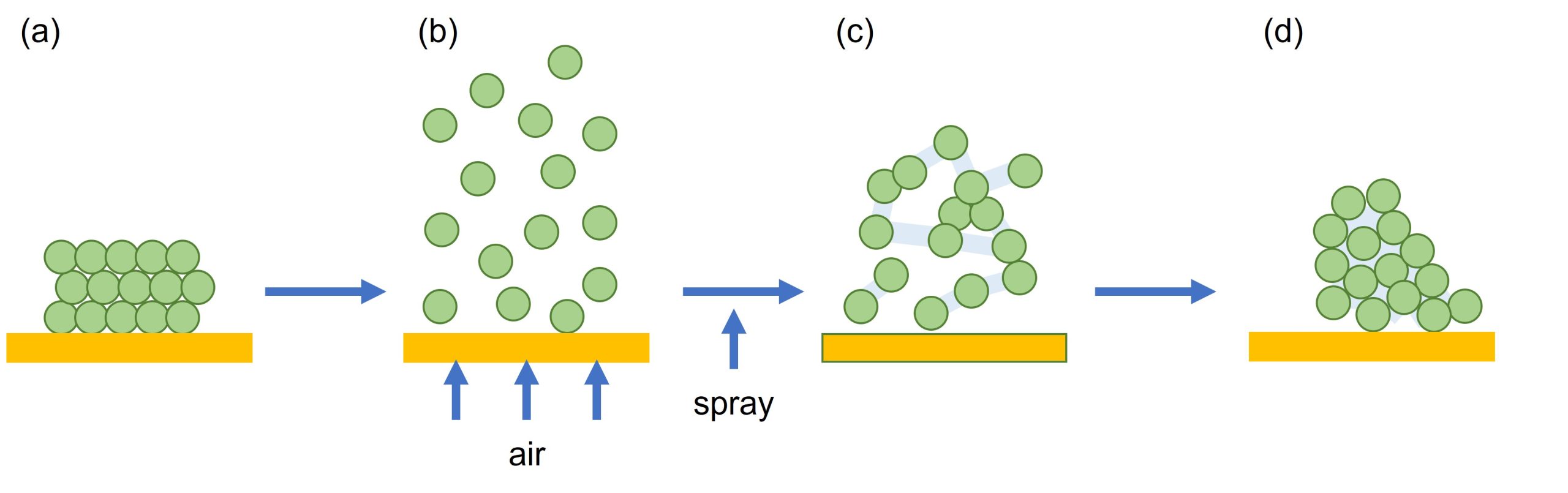
How can we help?
- By monitoring the rheological properties, it is possible to identify the end-point of wet granulation and reduce variations between batches, so improving quality control.
- Water can act as a lubricant, adhesive or a facilitator of granulation, therefore monitoring the powder’s response to water addition can reveal changes in physical properties as wetting occurs. By monitoring the flow properties of the wet mass in contrast to the dry powder, the effects of water or other solvents can be quantified.
2. Dry granulation
Dry granulation occurs when solid materials are combined to make granules without the addition of liquid and is generally used if a formulation is sensitive to moisture or heat. During this process the powder is often compressed using a roller (roller compaction) to form a ribbon, or can be compacted to form a ‘slug’, Figure 3. The ribbon is passed through mesh to form granules, whereas the slugs can be broken into smaller pieces using a hammer mill or passed through mesh. Further milling may be required to remove any lumps and generate a homogeneous powder.
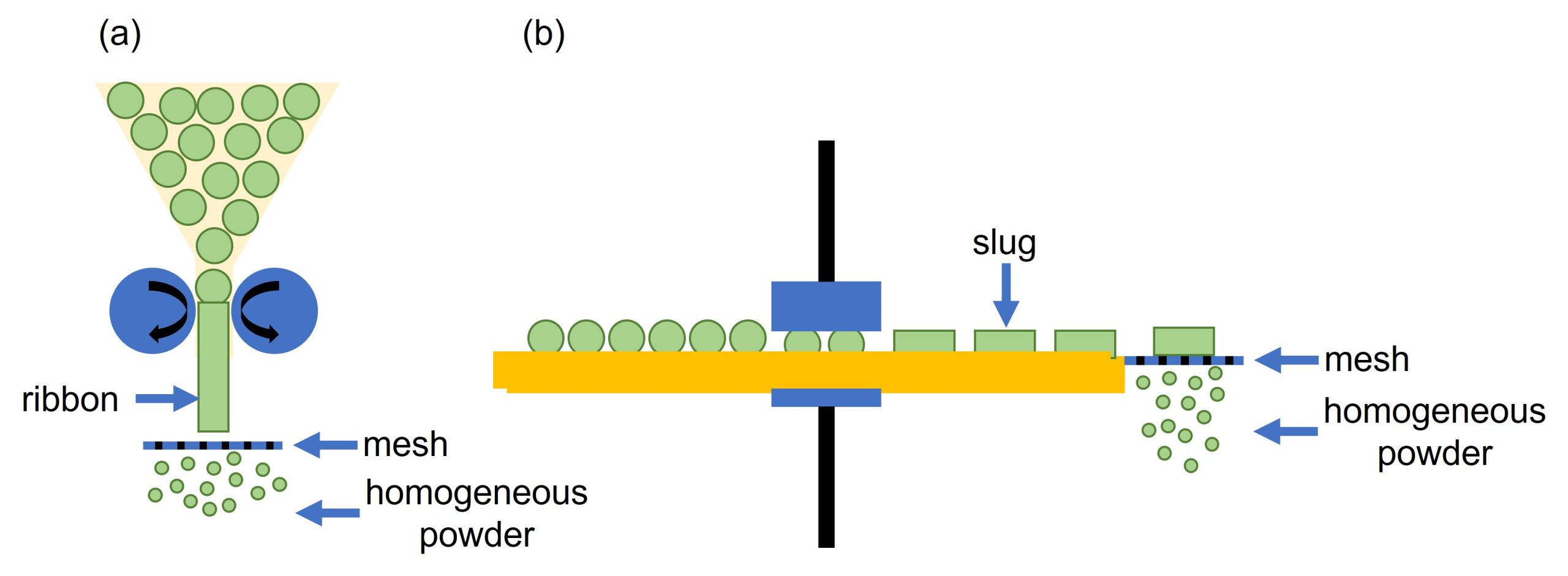
How can we help?
- Shear cell measurements can reveal how easily a powder flows, allowing comparison of flowability differences between powders with different particle sizes.
- Dynamic powder movement measurements are useful to probe powder behaviour once it is moving.
- Powder flow rheology can be used to investigate properties such as flowability when the powder is aerated or deaerated, or to identify troublesome behaviours such as rat-holing, arching and bridging.
- Measuring the stability of the powder, particularly when there is a large size-difference between the active and the excipient, can provide insight into long-term stability and propensity of powder separation upon storage.
3. Direct compression
Direct compression is for solids only. Samples are tumbled in a blender to make a homogenous powder that is then pressed into tablets, or the powder encapsulated, Figure 4.
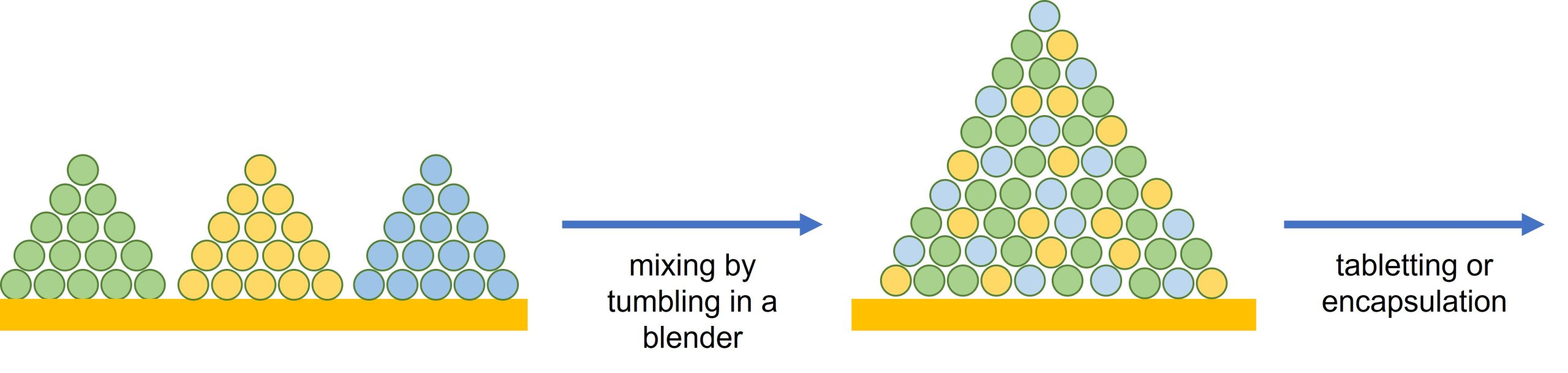
How can we help?
- Comparing the Flow Rate Index can reveal the impact of different glidants (powders added to aid flow) or the impact of different concentrations of glidant.
- Powder rheology can allow for interrogation of the relationship between the glidant and density, flow function, basic flow energy (BFE), specific energy and compressibility, which can all impact upon manufacture. BFE can also be used as a predictor of tablet cracking or capping during down-stream processing.
- Quantifying the degree of caking of a powder under differing conditions to probe optimal processability.
- Issues during final processing, for example powders self-ejecting from the tabletting machine, can be investigated through testing for low-stress flowability and powder permeability.
4. Particle coating
In this process, liquids and solids are combined and particles are sprayed with an atomised liquid, coating them in either sugar, a film or an enteric-coating. There are three usual methods for coating powders: by using a conventional coating pan; by using a fluid-bed; and by using a side-vented coating pan. The coating quality can be affected by a variety of factors, including atomisation of the coating material, tablet/powder shape, hardness of the tablet/powder and the equipment used.
When coating tablets or powders using a fluid-bed, the first stage is to fluidize the powder by aerating it under a constant flow of air, thus generating air-borne particles, Figure 5. These particles are then sprayed with the atomised liquid, covering the particles in a liquid layer. Once covered, the particles and dried, the fluidisation air-flow is stopped and the coated particles are discharged.
How can we help?
- Comparing the behaviour of the particles before and after coating can ascertain if coating is successful and how behaviours change.
- Assessing the aeration properties of the powder can probe fluidization properties and look into the quality of coating and ease of application.
- Assessing flow function of the powder in relation to the concentration of any additives can optimise time and costs.
- Looking at how other key variables affect flow properties, for example moisture content, consolidation, attrition and recyclability are all valuable and can impact upon processing and manufacture.
For further information about the testing services provided within this article, or if you have any questions, please feel free to get in touch.
