When you consider that the average bottle of body lotion or food dressing is knitted together with oil/water interfaces that can span a tennis court in area, it is not surprising that the strength, flexibility and robustness of those interfaces is critical for a stable and effective product. Interfacial rheology measurements shine a light on the physical properties of these interfaces and can prove valuable to the formulator and process designer looking to create a stable and satisfactory product.
What is Interfacial Rheology?

Interfacial rheology is the study of the flow and deformation of the interface between two immiscible fluids. Contrast this to its well-established big brother, “regular” rheology, where the behaviour of a bulk material is of interest. Interfacial rheology can aid process designers, formulators and chemists working on a huge number of products, from oil and gas to plant-based protein to household cleaning products.
Emulsions and foams are just two examples of when it is useful to measure the behaviour at an interface, where emulsions contain a liquid-liquid interface and foams a liquid-gas interface. In these cases, the interface is both mobile, deformable and potentially huge! An emulsion is formed by rapidly mixing oil and water to suspend one phase inside the other and a foam is formed by trapping gas bubbles inside a liquid. Both usually require the presence of a chemical agent to stabilise the product.
If you would like to arrange a discussion about how our characterisation capabilities can help you, please feel welcome to contact us.
Particle/Droplet size and Interfacial Area
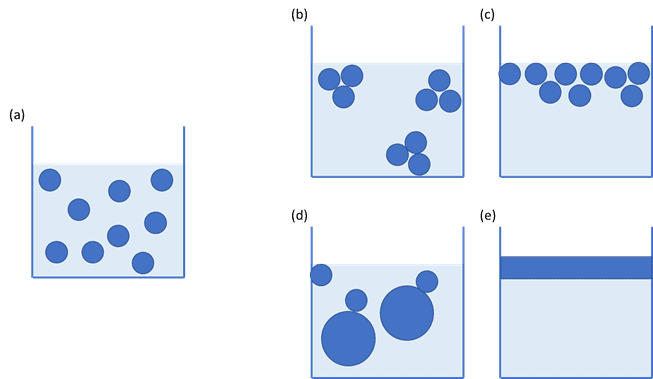
The size of droplets within an emulsion, or air pockets in a foam, can be affected by the speed of formation, the addition of stabilisers and the processing method used. Importantly, the particle size or droplet size of the dispersed phase can have a significant impact on the total surface area of the interface, which may then have an impact upon the stability of the product.
If an emulsion contains very small droplets, or a foam contains small pockets of air, the area of the interface can be huge and have a large bearing on the product’s behaviour. Measuring the properties of the interface can give valuable information to the formulator, saving valuable time and money.
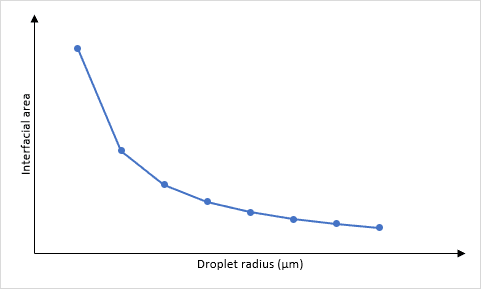
The size of the droplets and surface area are related because of the maths required when calculating the surface area and the volume. The easiest way of doing this is to imagine that each droplet is a spherical particle, therefore the surface area, S = 4πr² and the volume, V = πr³. The ratio of surface area to volume can be determined as:
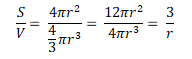
This means that a large droplet will have a large radius, r, and the surface area to volume ratio will be small. Conversely, a small droplet will have a small radius, r, and the surface area to volume ratio will be large.
Stability and the Interface
An interface in a foam or emulsion can be thought of as a dynamic, living entity with materials moving to and from the interface continually. Their stability is affected by the mechanical strength and viscoelasticity at the interface, the interfacial rheology. Foams and emulsions are not thermodynamically stable, and the dispersed phase will only remain dispersed for a finite period i.e. while the mechanical strength and viscoelasticity at the interface are favourable. Amending a formulation can affect these properties and may shorten the shelf-life of the material, leading to instability. Instability can lead to coalescence, flocculation, creaming or breaking down of the emulsion entirely.
Instability of foams can lead to collapse of bubbles (drainage) or coalescence. To summarise, the rate of breakdown in both foams and emulsions is controlled by interfacial tension, which can be measured using rheological techniques.
With this complex behaviour in mind, being able to tug, pull, deform, stretch and squash the interface to determine its properties, particularly in relation to shelf-life and stability, is an extremely powerful tool. There are two main testing regimes for doing this within interfacial rheology: dilatational and shear rheology
Dilatational Interfacial Rheology
Dilatational interfacial rheology is where a droplet of liquid is suspended from a capillary and pulsated to increase and decrease the surface area of the droplet. This pendant droplet can either be suspended in the air or within another bulk liquid. As the droplet is expanded, there is a rush of ingredients to the freshly created surface. As the drop-size is decreased, those ingredients are squeezed away from the surface.
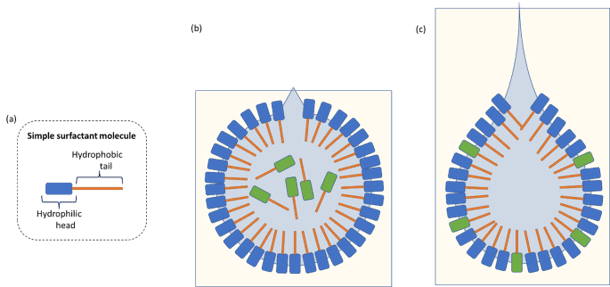

Pulsation affects the equilibrium of materials encapsulated within the liquid droplet and affects the chemical composition of materials at the interface. Unsurprisingly, the physical properties of materials within the droplet can have a marked effect on the interfacial behaviour. For example, if large polymeric materials are encapsulated then chain entanglement can lead to slowing of reorganisation at the interface, and therefore affect the interfacial behaviour or stability.
Dilatational rheology
Dilatational rheology is especially useful when looking at the stability of an emulsion over time or when under stress, for example within coffee creamers or in the microencapsulation of fragrances and/or flavours. In some cases, however, the interface formed is rather rigid and cannot be easily pulsated, or the interface under study is a film. In these cases, a shear rheology technique should be used.
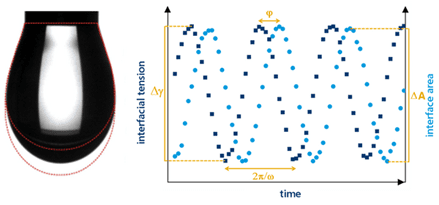
Interfacial Shear Rheology

As with dilatational interfacial rheology, interfacial shear rheology can also look at both the liquid-liquid and the liquid-gas interface. In this technique the interface area is kept constant but it is deformed in shear to measure the surface stress. Measuring stress at the interface is achieved by using a du Noüy ring. During the shear rheology test, a du Noüy ring is lowered so that it is in contact with the denser phase; the less-dense phase is usually air. In the classic interfacial experiments using a du Noüy ring, the ring is lifted through the less-dense phase by applying axial force. Providing the attractive forces between the ring and the dense phase are strong, the denser phase will be pulled up through the less-dense phase to give an ‘elongated’ dense phase. This can then up be oscillated up and down to provide data about the interface. As with dilatational rheology, this pulsation affects the equilibrium of materials within the bulk liquid phase, affecting the chemical composition of materials at the interface. A relatively new innovation is to oscillate the du Noüy ring side-to-side, in parallel with the interface itself. This also provides valuable data on the stability of the interface and can be a valuable complimentary technique when vertical deformation is not the primary shear applied during use or processing of the material or interface.
Which is best for my needs? To summarise, interfacial rheology provides formulators with key information relating to stability of a formulation and R&D specialists with vital guidance for next steps. It helps to keep cosmetic emulsions stable and vegan milk foams frothy. Dilatational rheology is useful when a material is encapsulated within a droplet and either suspended in air or another liquid, and shear rheology when a film is formed at an interface either with air or another liquid.
Contact us now to discuss your specific challenges
