We provide a fast-turnaround surface tension testing and wetting analysis service for your samples.
We can provide measurements of surface tension and hydrophobicity or hydrophilicity of a surface or liquid as a function of time and/or temperature. On completion of our testing you will receive a full written report with graphical and numerical results and explanatory notes.
We are an expert measurement lab and any support you may need from us regarding the relevence, interpretation or application of the results we provide is all included in the initial cost of the testing.
Get a quick quote
Surface tension measurements for wetting properties
From simple measurements of equilibrated surface tension, to more complex time dependent or temperature dependent relationships, we have a host of accessories which enable us to tightly control the conditions of measurement for your sample. For short time-scale processes we can observe up to a resolution of 5ms.
Interfacial Rheology Measurement
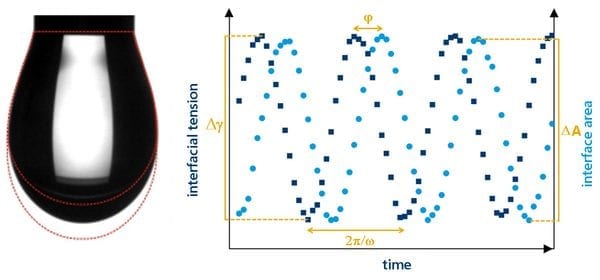
Interfacial rheology is an exciting and relatively new technique that enables the characterisation of viscoelastic properties of an interface such as modulus and stress relaxation. These properties arise from the time-dependent interchange of surface active entities between the bulk solution and the interface. As interface is created surfactants take a finite time to adsorb to an equilibrium condition; if the interface is then compressed those surfactants are then forced back into the solution.
By oscillating the size of a pendant drop and measuring the interfacial tension throughout it is possible to characterise these processes. This method is known as dilatational interfacial rheology.
Contact us now to discuss your specific challenges.