
If you are currently relying on pendant drop analysis or force tensiometry to measure surface tension for your nasal spray formulations, you will be missing critical insights relevant to highly dynamic atomisation processes. Furthermore, viscosity measurements can prove difficult due to their low viscosity, potential non-Newtonian shear-thinning behaviour, and the requirement to measure at the higher shear rates relevant to atomisation.
At the Centre for Industrial Rheology, we address these challenges with two key characterisation services that deliver detailed insights relevant for spraying and atomisation.
Contact us now to speak with the experts
Bubble Pressure Tensiometry – This enables the measurement of surface tension at short surface ages, from 10 milliseconds. These timescales are directly relevant for spraying processes, where new surfaces are created rapidly, requiring surfactants to diffuse quickly to newly formed interfaces.
Rheology – Our suite of high-performance research rheometers offers the sensitivity, speed, and torque range required to characterise low viscosity formulations, as well as attaining higher shear rates more relevant to spraying processes. This is particularly relevant if thickening or mucoadhesive polymers are incorporated within a formulation.

Together, these advanced characterisation techniques can deliver a more accurate and application-relevant understanding of nasal spray formulations.
Advanced Testing for Nasal Spray Formulations
Among their many applications, nasal sprays are commonly used to deliver medication directly to the nasal passages to reduce inflammation. The efficacy and overall consumer experience of a nasal spray is not solely determined by the active ingredients used. The addition of surfactants, such as benzalkonium chloride and polysorbate 80, can directly impact how a spray is generated and deposited within the nasal cavity.
In this study, we sought to characterise three different nasal sprays using our advanced characterisation techniques.
- Boots Decongestant Nasal Spray
- Sinex Micromist Nasal Spray
- Otrivine Congestion Relief Nasal Spray
Both Boots Decongestant and Sinex Micromist contained 0.005% of oxymetazoline hydrochloride as the active ingredient, while Otrivine congestion relief contained 0.1% of Xylometazoline. 
Viscosity as a function of shear rate, and surface tension at short surface ages were characterised to identify any differences between samples and assess how these differences may translate to efficacy and consumer experience.
Surface Tension Characterisation at Short Surface Ages
Figure 1 shows the extended surface age range that is not characterisable through optical and force tensiometry, highlighting the insights that are missed when not employing bubble pressure tensiometry.
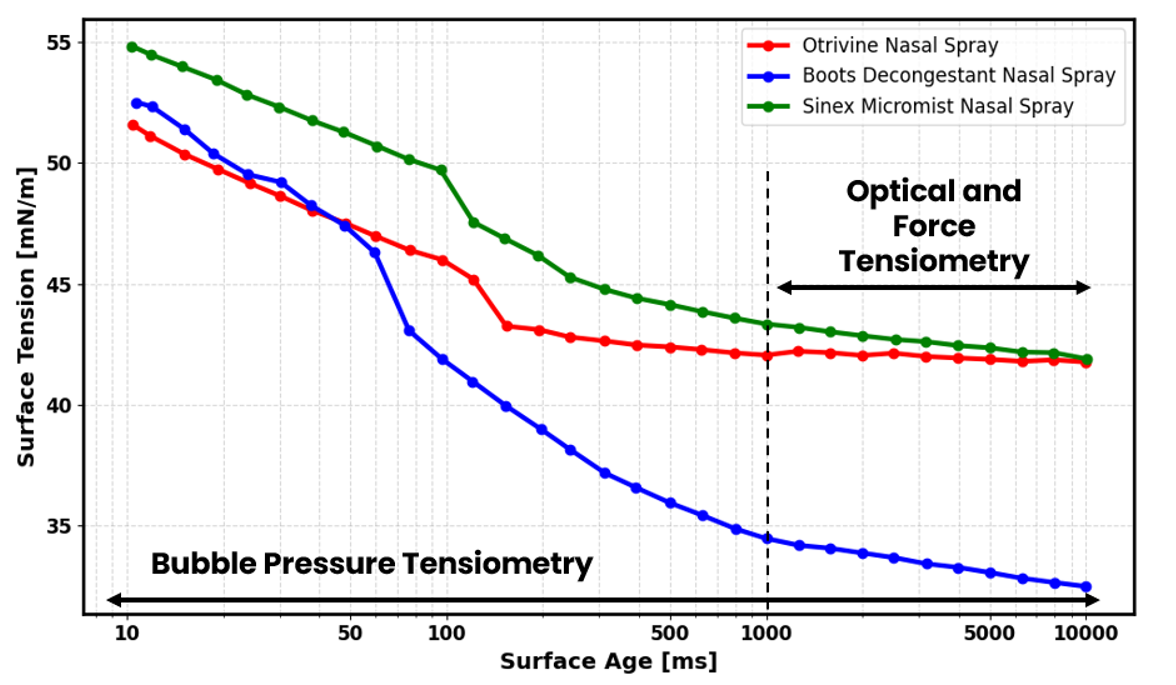
An ideal nasal spray formulation should exhibit a high surface tension at 10ms as this results in increased capillary forces at the liquid-air interface. This ensures that the meniscus at the nozzle remains stable, allowing controlled droplet formation. However, surface tension should then decrease rapidly to enable effective wetting on nasal surfaces and promote further droplet breakup.
The results obtained demonstrate clear differences in dynamic surface tension behaviour between the three samples. At a surface age of 10ms, all samples maintain values above 50 mN/m, with Sinex Micromist exhibiting the highest value at 54.85 mN/m. This is consistent with the requirement for preventing premature meniscus destabilisation at the nozzle.
Boots Decongestant exhibits the most pronounced reduction in dynamic surface tension, reaching an equilibrated value of 34.5 mN/m. This reduction in dynamic surface tension reflects the mobility of surfactants present within the formulation and can aid in subsequent wetting of nasal surfaces. In contrast, both Otrivine and Sinex equilibrate at values of around 42 mN/m, suggesting less effective wetting compared to Boots.
Viscosity
Ensuring you have the optimal viscosity is essential for spray formulations. When viscosity is below 3 × 10-3 Pa·s, viscous contributions to spraying are minimal [1]. In this case, surface tension effects dominate and heavily influence the final droplet size.
However, if viscosity is above this value, then viscous effects become more significant. This affects sprayability through suppression of Rayleigh instabilities and inhibition of droplet-pinch off, leading to poorer atomisation.
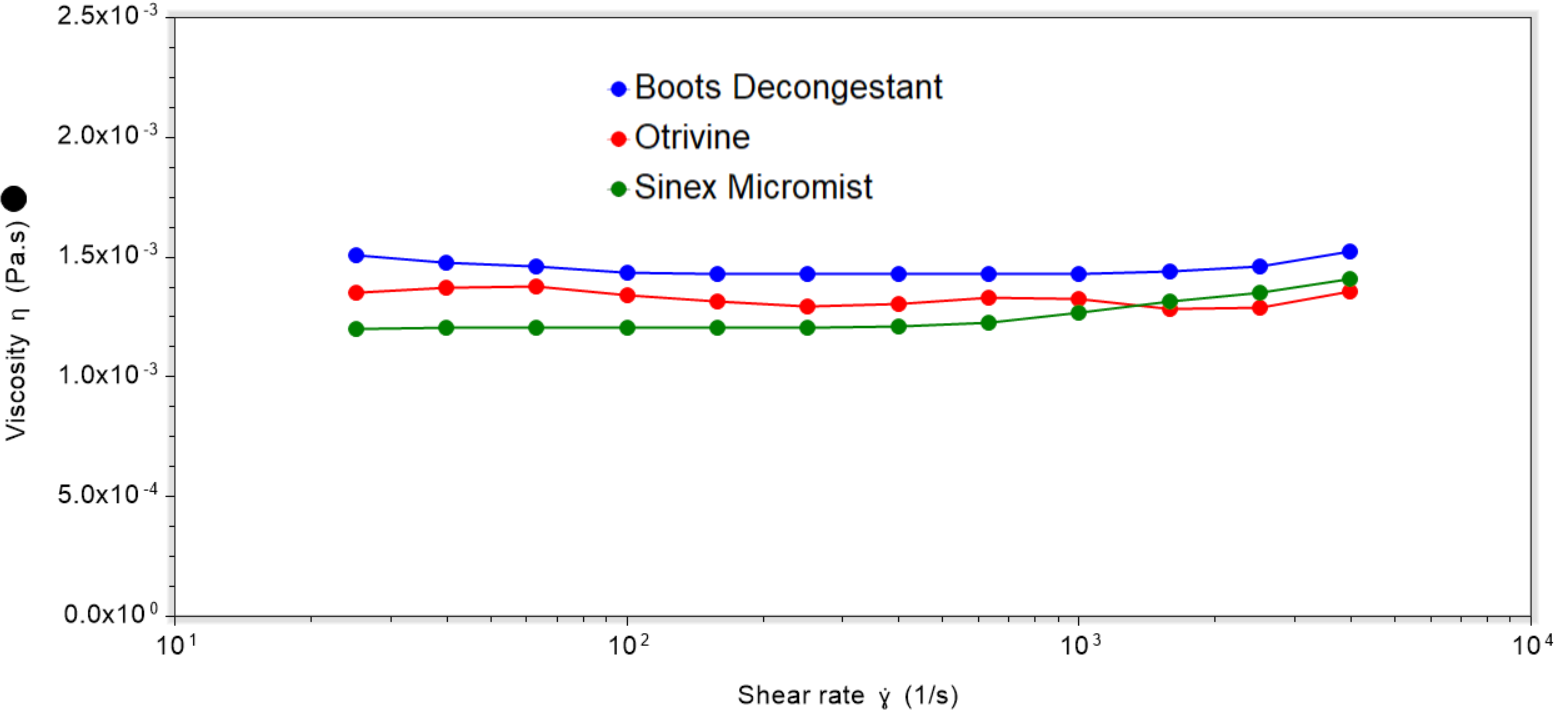
The results captured show that all samples display similar viscosity values around 1× 10-3 Pa·s, with Newtonian behaviour. The small increases observed at higher shear rates occur due to turbulence or secondary flows, and are a measurement artefact. These low viscosity values suggest that spraying behaviour is primarily governed by surface tension rather than viscosity for this sample set.
It should be noted that the addition of polymeric thickeners or other rheology modifiers can cause the sample to exhibit non-Newtonian, shear-thinning behaviour. This is where viscosity decreases as the shear rate increases. In such cases, attaining higher shear rates representative of spraying processes is important to ensure that viscosity is sufficiently low such that sprayability is not adversely affected.
Predicting Droplet Size in Nasal Spray Formulations
We can approximate droplet sizes across the tested formulations to assess any differences between samples using empirical correlations. The analysis conducted in this study follows the methodology outlined by Sijs et al., which is applicable where viscous effects are minimal, with surface tension values used at 20ms [2].
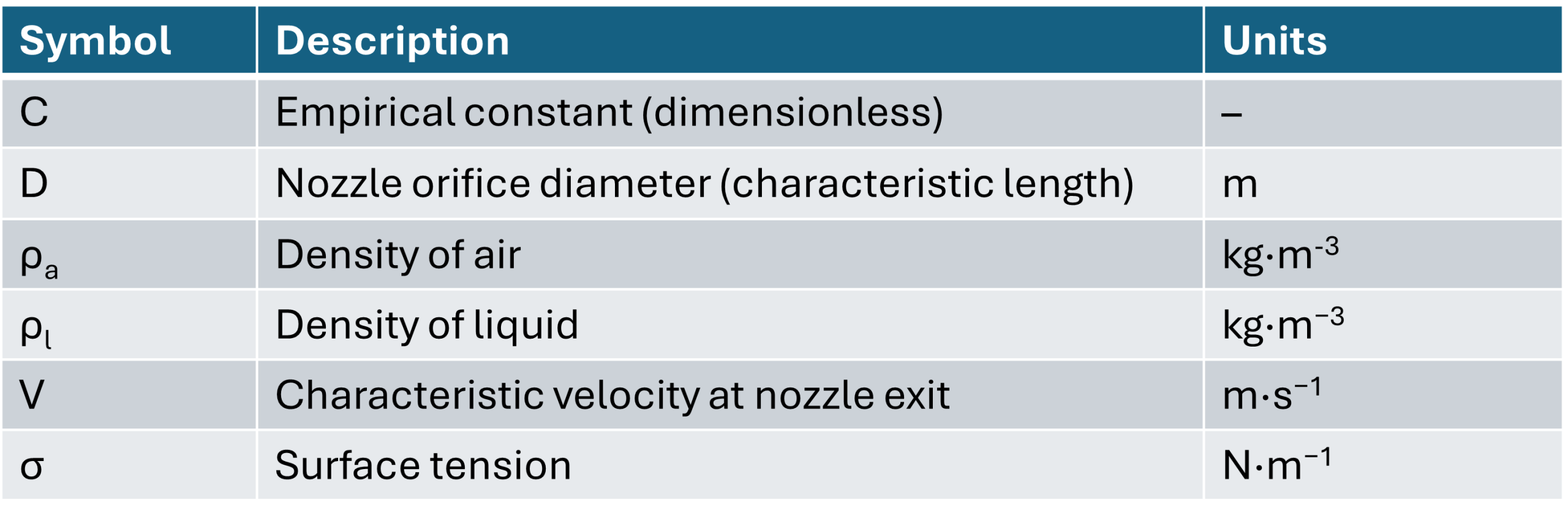
Firstly, we calculate the Weber number, We, which describes the balance between inertial and surface tension forces.
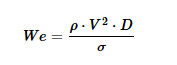
For these calculations, velocity at the nozzle exit and orifice diameter are kept constant across formulations in order to isolate the effect of formulation properties alone. It must be noted that the values of nozzle diameter and liquid velocity are estimates based on literature values [3,4]. Nozzle design will also naturally influence spray dynamics; however, that is not the focus of this study.
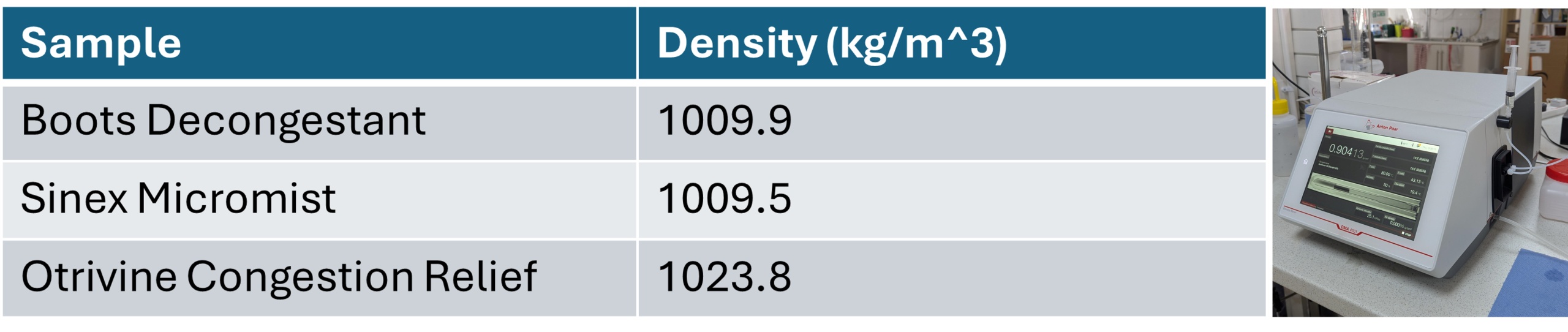
Density values were measured with our density meter, obtaining the following values:
Following the calculation of the Weber number, mean droplet sizes can be predicted using the empirical correlation:
![]()
where,
![]()
The constant C accounts for effects not captured by the Weber number, with a value of 1.95 used by Sijs. The estimated droplet sizes obtained are as follows:
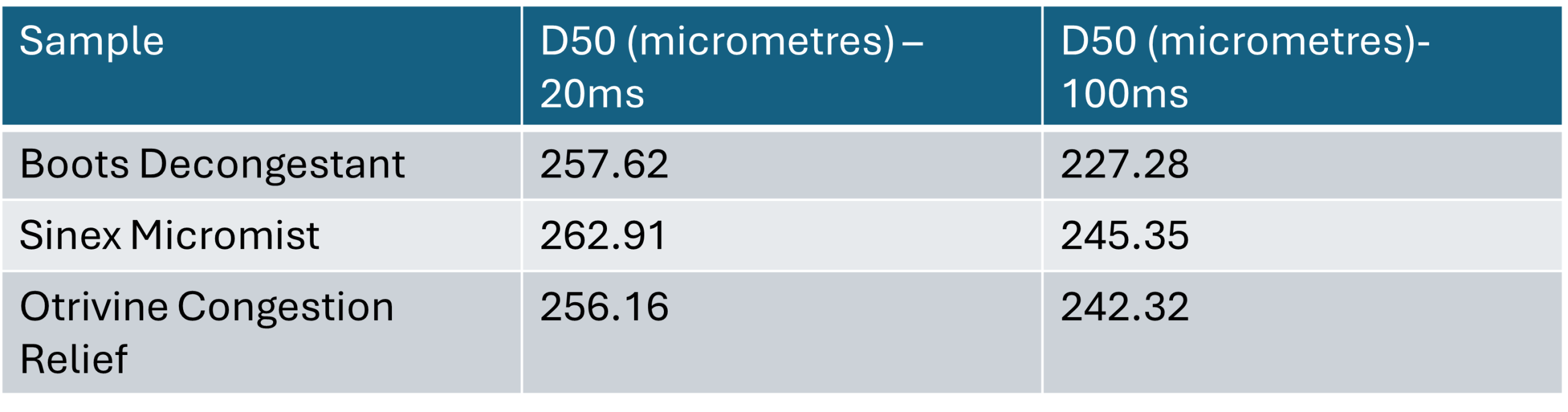
The calculations demonstrate that when surface tension at 20ms is used, the predicted mean droplet size is larger than when surface tension at 100ms is used. Specifically, the mean droplet size increases by 13.3% for Boots Decongestant, 7.2% for Sinex Micromist, and 5.7% for Otrivine Congestion Relief.
While the differences in droplet sizes between the three products appear modest, the variation introduced by using surface tension values at different surface ages can be seen as significant. Since droplet formation occurs within 20 milliseconds, utilising surface tension at longer ages will not accurately predict real droplet sizes.
Overall, these results for droplet size provide a simplistic view of a spraying process and do not consider meniscus stabilisation at the nozzle, droplet pinch-off and whether effective wetting occurs on the substrate. A full view of how surface tension evolves at short surface ages is required to understand these parameters.
Summary
This study has demonstrated the value of bubble pressure tensiometry to generate crucial insights into dynamic surface tension behaviour, offering a more comprehensive understanding of atomisation. Beyond nasal sprays, this technique may also have relevance to other consumer health and personal care applications, including formulation of throat sprays, antiperspirants and foaming cleansers, and the development of spray-drying processes.
If you are looking to enhance your understanding of your formulations to optimise properties such as sprayability and wetting, do not hesitate to contact us to see how we can support you.
References
[1] – Chideme N, De Vaal PL. Effect of liquid viscosity and surface tension on the spray droplet size and the measurement thereof.
[2] –SIJS ET AL” with “Sijs R, Kooij S, Denn MM, Villermaux E, Bonn D. What determines the drop size in sprays?. Physical review X. 2018 Jul 1;8(3):031019.
[3] – Zeng X-M, Walsh D, Ly J-C-Y, Morales A. Nasal spray device. US patent application publication US20120085345A1, published 12 April 2012.
[4] – Liu X, Doub WH, Guo C. Evaluation of droplet velocity and size from nasal spray devices using phase Doppler anemometry (PDA). International journal of pharmaceutics. 2010 Mar 30;388(1-2):82-7.

Related Articles;
Dynamic Surface Tension Characterisation at Short Surface Ages
Wasif Altaf serves as an Applications Specialist at the Centre for Industrial Rheology, leveraging a chemical engineering background (BEng) to bridge theory and practice. His work focuses on advanced rheological characterisation.
