
Utilising our TA-XT Plus universal tester and texture analyser, we can precisely measure mechanical responses under a wide variety of controlled deformation and failure conditions. This article showcases our diametral crush test capabilities for pharmaceutical tablets, providing comprehensive insights into material properties that may go unnoticed in conventional testing methods.
Beyond evaluating the mechanical properties of the tablets themselves, we examined how humidity affects a tablet, as well as extending our capabilities to conduct mechanical testing for packaging.
Contact us to discuss mechanical testing
Understanding Tablet Strength for Pharmaceutical Formulation
The formulation of pharmaceutical tablets involves balancing several critical attributes to ensure viable drug delivery whilst maintaining tablet structural integrity. A pharmaceutical tablet is generally required to possess sufficient hardness to ensure that it does not break during handling, processing, packaging, and transportation.
Traditional tablet strength tests typically only measure the peak force required for fracture, potentially overlooking crucial characteristics. To address this limitation, we can employ a diametral crush test to provide comprehensive insights by examining tensile failure modes.
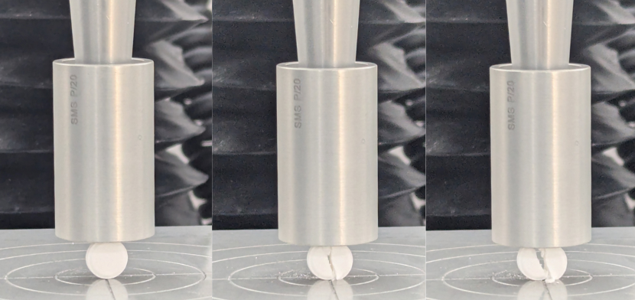
Although this test is done in compression, it can be observed that tablets typically fail in tension along their diameter, perpendicular to the compressive force applied. The measured force at fracture can be converted to stress and strain values, revealing mechanical properties such as:
- Tensile Strength The force data collected can be converted to tensile stress values using:
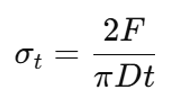 where F is force (N), D is diameter (m), and t is thickness (m). The tensile strength is the maximum tensile stress that the tablet can withstand.
where F is force (N), D is diameter (m), and t is thickness (m). The tensile strength is the maximum tensile stress that the tablet can withstand. - Work of Failure – the total energy absorbed by the tablet until fracture occurs. This is the area under the force-time curve.
- Tablet Brittleness Index – a measure indicating susceptibility to brittle fracture. This is the reciprocal of the strain at the fracture point, with a lower strain at fracture indicating that the tablet is more brittle.
- Hardness – the force required for the initial fracture to occur.
Evaluating these properties provides critical insights into the tablets’ internal bonding quality, compaction efficiency, and fracture mechanisms.
Insights from Diametral Crush Testing for Pharmaceutical Tablets
Tablets were crushed with a 20mm cylinder probe at a compression rate of 0.03 mm/s. The tester was fitted with a 50kg load cell. Initially, three different tablets were tested using this technique.
- Multi Vitamin Tablet
- Biotin Hair Tablet
- Allergy Tablet
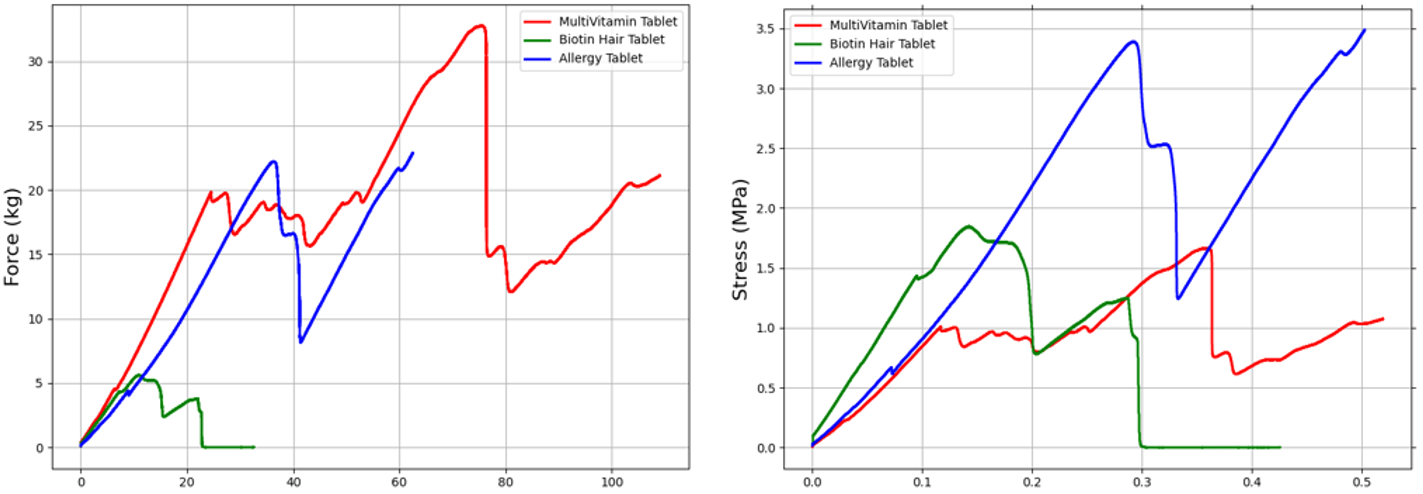
The results show a comparative analysis of the measured force responses of the tablet samples. The Force vs. Time graph shows the load each tablet experiences throughout testing, highlighting differences in their ability to withstand applied compressive forces. By accounting for the geometry of each tablet, the force data can be further normalised into stress and strain data.
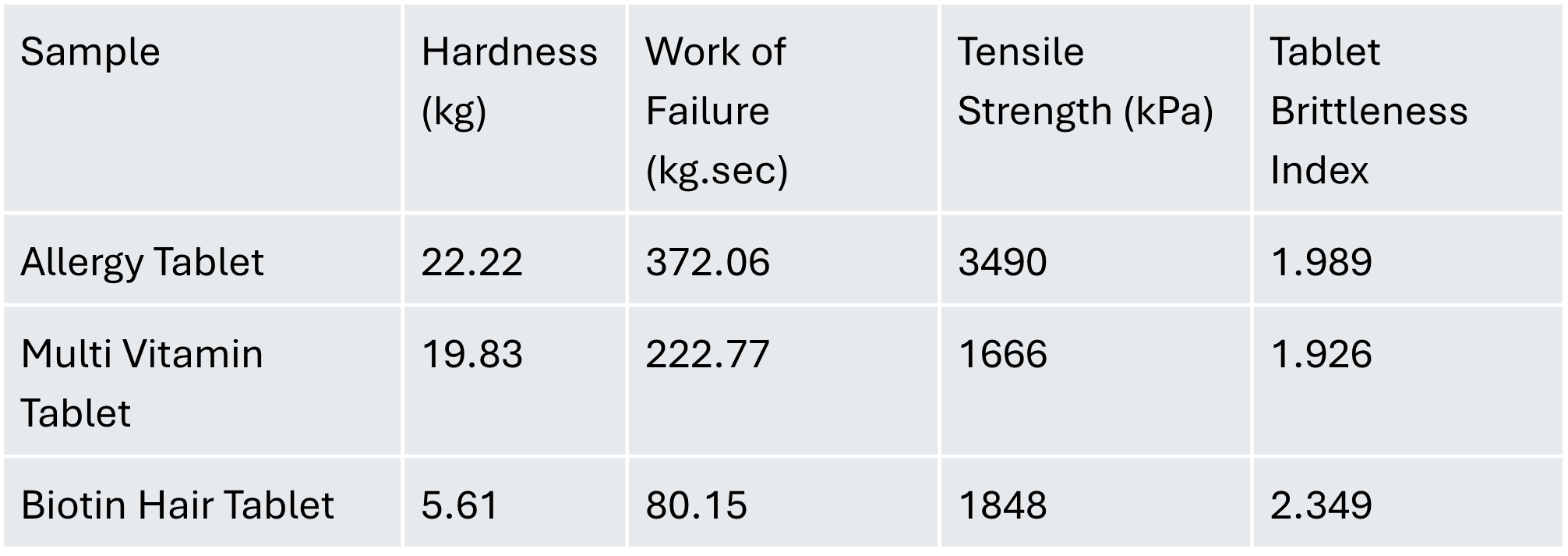
The allergy tablet exhibited the highest tensile strength, hardness, and work of failure. These characteristics indicate that the allergy tablet possesses robust structural integrity, requiring significant force to fracture. Such mechanical strength suggests the tablet is well-suited for harsh process and transport conditions.
While the multi-vitamin tablet had moderate hardness and work of failure, it showed the lowest tensile strength among the samples. This indicates that, although relatively resistant to compression initially, it may fracture more readily under tensile stresses.
The Biotin hair tablet, notably weaker, displayed the lowest hardness and work of failure, with an intermediate tensile strength. Its higher tablet brittleness index underscores its susceptibility to brittle failure, necessitating careful handling and specialised packaging to avoid damage during processing and transportation.
Collectively, hardness, work of failure, tensile strength, and brittleness provide critical insights into each tablet’s mechanical performance. Understanding these factors allows for targeted improvements in formulation, ensuring tablets achieve the ideal strength without compromising drug efficacy.
Diametral Crush Testing for Humidified Pharmaceutical Tablets
At the Centre for Industrial Rheology, we are equipped with humidity and environmental control chambers that facilitate comprehensive thermal and humidity ageing studies. These capabilities allow us to simulate conditions that materials may encounter during manufacturing, handling, and transport. Conducting such studies ensures a thorough understanding of how environmental conditions influence a material’s properties.
Further analysis was conducted to evaluate the effects of humidity ageing on the allergy tablets’ mechanical properties. The tablets were subjected to an environment of 80% relative humidity at 20°C for 6 days and showed substantial changes in their stress-strain behaviour compared to fresh tablets, as expected with such humid environments.
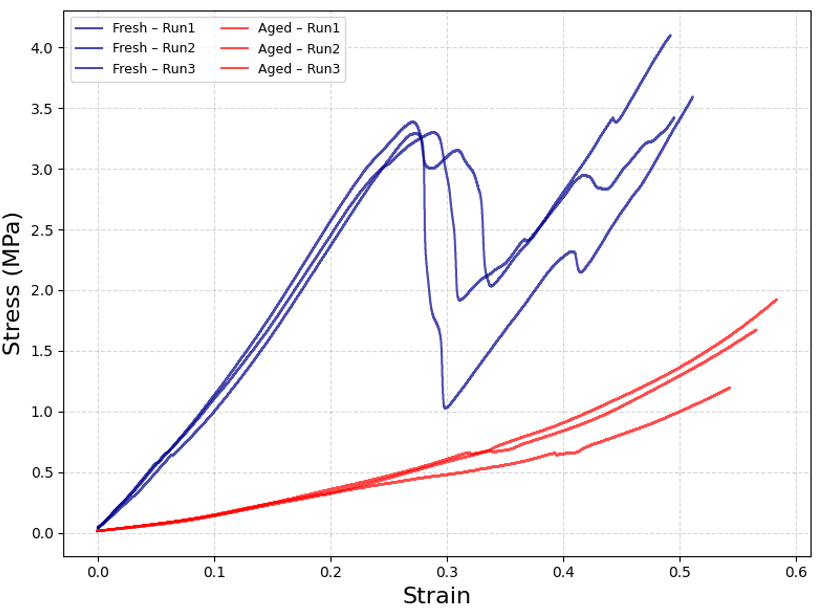
As previously observed with the allergy tablets, when freshly opened, they exhibit identifiable fracture points. In contrast, the tablets that had been exposed to humid conditions exhibited significant differences in their mechanical properties. The stress-strain curves for the humidity-aged tablets no longer display a clear fracture point. Instead, stress increases gradually with strain, revealing the material’s transition from a rigid structure to a softer, easily crushable structure. This softening effect underscores the need to ensure that these tablets are not exposed to harsh conditions.
In addition to looking at the effect of these conditions on the tablet itself, we can age the tablets within their packaging. This confirms whether the packaging used is sufficient to shield the tablet from any harsh environments it may encounter and can serve as an aid for optimal packaging selection.
3-Point Bend Testing for Pharmaceutical Tablet Packaging
The previous results revealed that the Biotin hair tablet possessed the lowest hardness, prompting us to investigate whether there were any differences in the packaging of this tablet to compensate for its softer structure. We conducted a 3-point bend test on each tablet’s packaging to determine if there were variations in packaging strength. Tests were run up to 5% strain, calculated based on the geometry of each sample.
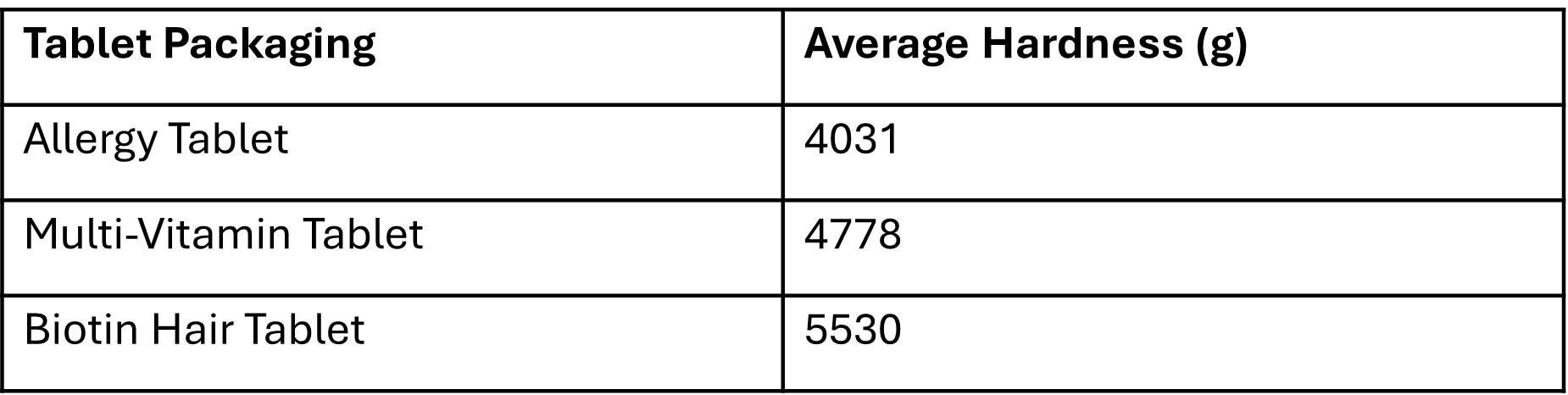
The results show that the Biotin hair tablet packaging exhibits the highest hardness, followed by the multi-vitamin and allergy tablet packaging, respectively. This suggests that the design of each tablet’s packaging has been taken into consideration based on its individual mechanical properties. Notably, the stronger packaging of the Biotin hair tablet may serve to compensate for its lower tablet hardness, providing more protection during handling and transportation.
Summary
This study demonstrates the value of advanced mechanical testing for pharmaceutical tablets. By moving beyond simple fracture testing, we provide a more nuanced view of tablet integrity through metrics such as tensile strength, work of failure, and brittleness index. Our ability to assess packaging through methods such as the 3-point bend test allows a complete evaluation of both the tablet and its packaging. With our TA-XT Plus Universal Tester and controlled environment chambers, we offer a comprehensive suite of testing solutions that provide detailed material insights.

Related Articles;
Powder Flow Rheology of Oral Solid Dosage Pharmaceuticals
Predicting Caking of Paracetamol Powder Using Dynamic Powder Flowability Testing
Wasif Altaf serves as an Applications Specialist at the Centre for Industrial Rheology, leveraging a chemical engineering background (BEng) to bridge theory and practice. His work focuses on advanced rheological characterisation.
